FDA Authorization Pfizer-BioNTech COVID-19 Vaccine
Il vaccino di BioNTech/Pfizer (Comirnaty) è il primo ad essere in via definitiva, dopo l'uso in fase "emergenziale" dalla FDA (in America)
L'approvazione è per tutte le persone di oltre 16 anni in su.
Termina quindi fase "emergenziale" dell’uso ed entra nell’iter normale di commercializzazione e utilizzo.
FDA, 11 Dec 2020
Download - FDA Pfizer BioNTech COVID-19 Vaccine Letter of Authorization
On December 11, 2020, the U.S. Food and Drug Administration issued the first emergency use authorization (EUA) for a vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. The emergency use authorization allows the Pfizer-BioNTech COVID-19 Vaccine to be distributed in the U.S.
- Emergency Use Authorization Status: Authorized
- Name: Pfizer-BioNTech COVID-19 Vaccine
- Manufacturer: Pfizer Inc.
Authorized Use
For the prevention of 2019 coronavirus disease (COVID-19) for individuals 16 years of age and older
Common Side Effects
The most commonly reported side effects, which typically lasted several days, were pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, and fever. Of note, more people experienced these side effects after the second dose than after the first dose, so it is important for vaccination providers and recipients to expect that there may be some side effects after either dose, but even more so after the second dose. Learn more.
..
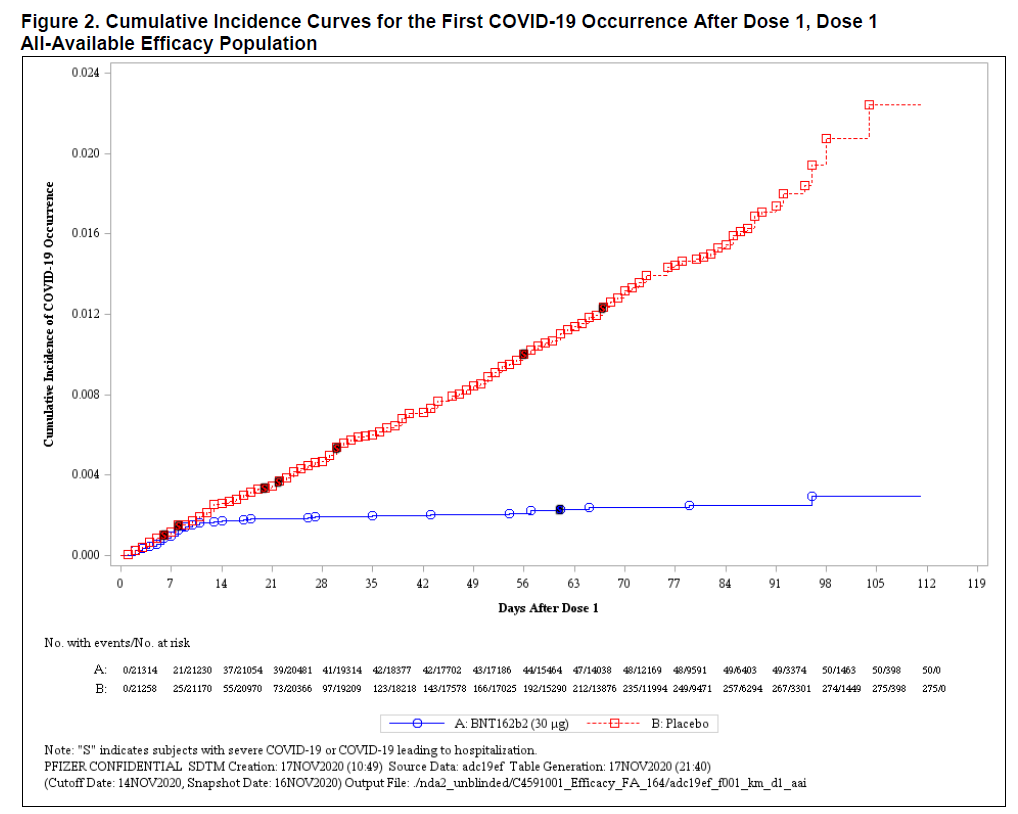
Cumulative Incidence Curvers for the Firt COVID-19 Occorrence After Dose 1 (FDA Briefing Document)
Il grafico ha 2 curve, la prima azzurra rappresenta il numero di persone che hanno ricevuto una dose di vaccino al giorno 0 fino al 112 e l’altra rossa rappresenta il numero di persone che hanno ricevuto un vaccino placebo (nello stesso periodo).
Dal grafico si evince, che, fino a 14 giorni, le curve sono quasi sovrapponibili: 21 casi tra i vaccinati e 25 casi tra quelli non vaccinati. Da questo punto il sistema immunitario dei vaccinati comincia a produrre anticorpi contro COVID-19. Le curve dei due gruppi (nessuno dei partecipanti sa a quale gruppo appartiene) divergono notevolmente.
Tra i vaccinati, dal giorno 14 fino al 112 (arco temporale di 3 mesi e 22 gg), si ammalano solo altre 29 persone; tra i non vaccinati se ne ammalano altri 250 nello stesso arco temporale di somministrazione. E' stata somministrata una sola dose.
FDA
Collegati