ISO 14708-1:2014 / Active implantable medical devices (AIMD): Safety, marking and information
ID 19311 | 26.03.2023 / Preview in allegato
ISO 14708-1:2014
Implants for surgery - Active implantable medical devices - Part 1: General requirements for safety, marking and for information to be provided by the manufacturer
Data pubblicazione: 2014
_______
ISO 14708-1:2014 specifies requirements that are generally applicable to active implantable medical devices. The tests that are specified in ISO 14708 are type tests and are to be carried out on samples of an active implantable medical device to show compliance. ISO 14708-1:2014 is applicable not only to active implantable medical devices that are electrically powered but also to those powered by other energy sources (for example by gas pressure or by springs).
ISO 14708-1:2014 is also applicable to some non-implantable parts and accessories of the active implantable medical devices.
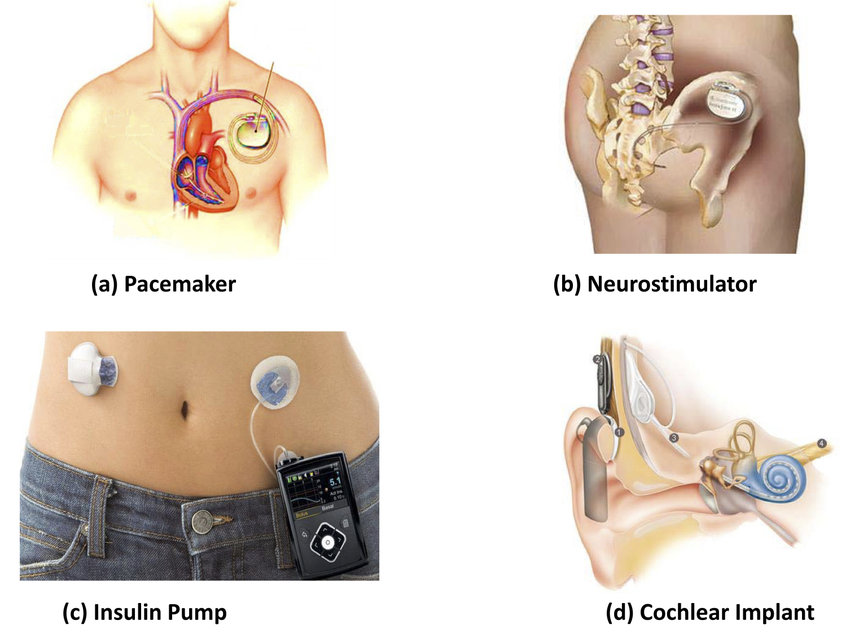
Example Active implantable medical devices
Collegati
MDR Regolamento dispositivi medici | Reg. (UE) 2017/745
Il Regolamento Dispositivi Medici (UE) 2017/745 - (MDR)
Norme armonizzate Regolamento dispositivi medici (MD) 2017/745/UE
Il Regolamento Dispositivi Medici (UE) 2017/745 - (MDR)
Regolamento (UE) 2017/745 MDR: Persona responsabile rispetto normativa (PR)
CEI EN 50527-1 Procedura Valutazione esposizione CEM lavoratori con dispositivi medici impiantabili attivi (DMIA)
Valutazione del rischio EMC per i lavoratori portatori di pacemaker
CEI UNI EN ISO 14708-2:2023
ISO/TR 14283:2018




















